Our neurostimulation technology addresses critical unmet medical needs
by providing solutions for key medical conditions.
Scroll down
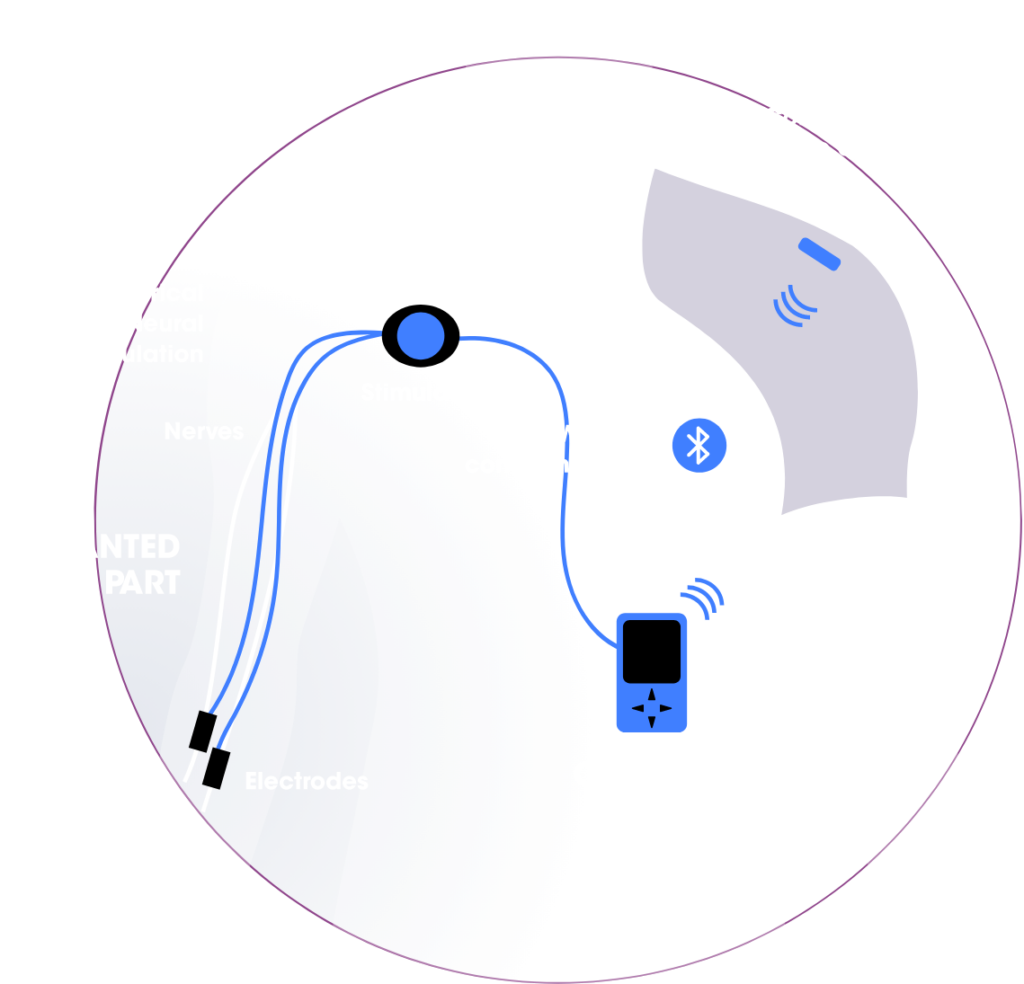
We are developing implantable neurostimulation solutions that enable patients with complete tetraplegia to regain upper limb autonomy.
To achieve this, we are creating the first neurostimulation device capable of inducing selective activation of targeted muscles by steering electrical currents through one or more nerves.
In the near future, patients will be able to recover functional use of one hand, allowing them to perform everyday activities such as grasping objects or eating with a fork. Looking ahead, we plan to expand the application of our neurostimulation technology to treat a variety of other pathologies and motor impairments.
The effectiveness of Neurinnov’s approach has been successfully demonstrated in a preliminary clinical study, with results published in Nature Scientific Reports.
Neurinnov, a spin-off from INRIA, the French national institute for research in digital science and technology, and the University of Montpellier, was founded in 2018 by David Andreu and David Guiraud. With over 20 years of research and expertise in micro-electronics, software engineering, electrophysiology, and functional physiology, the company is at the forefront of innovation in healthcare. Headquartered in Montpellier, France, Neurinnov has developed a versatile technology platform that creates value across multiple pathologies, addressing critical unmet medical needs.
Contact
Phone: +33 (0)4 34 34 80 28
Email: contact@neurinnov.com